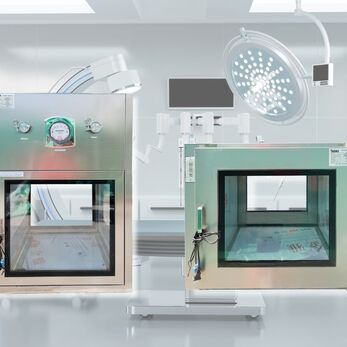
Dynamic Passbox is an important device in the pharmaceutical cleanroom system, helping to transfer items between areas with different levels of cleanliness while maintaining a sterile environment. Dynamic Passbox not only removes dirt and microorganisms but also prevents the risk of cross-contamination.
- 1. What is a Dynamic Passbox?
- 2. Why is a Dynamic Passbox essential in pharmaceutical production?
- 3. Benefits of using Dynamic Passbox in pharmaceuticals
- 4. Where is the Dynamic Passbox suitable within a pharmaceutical facility?
- 5. Key Criteria for Selecting a Dynamic Passbox
- 6. Frequently Asked Questions about Dynamic Passboxes in Pharmaceuticals
- 7. Need Help Choosing the Right Dynamic Passbox for Your Pharmaceutical Facility?
1. What is a Dynamic Passbox?
A Dynamic Passbox is an intermediate device commonly used in pharmaceutical cleanrooms, allowing materials to be transferred between two areas with different cleanliness levels or pressures without compromising the controlled environment.
What makes the Dynamic Passbox unique is its integration of HEPA/ULPA filters and a recirculating air blower inside the chamber, which helps remove dust and microorganisms before, during, and after material transfer. This not only ensures a clean airflow but also significantly prevents cross-contamination in pharmaceutical manufacturing processes compliant with GMP, EU-GMP, or PIC/S standards.
Distinguishing Dynamic Passbox vs. Static Passbox
|
Criteria |
Dynamic Passbox |
Static Passbox |
|
Air filtration |
HEPA/ULPA filter + air blower |
None |
|
Application |
Transfers between areas of different cleanliness levels |
Transfers between areas of same cleanliness level |
|
Cost |
Higher |
Lower |
|
Purpose |
Clean airflow - control cross-contamination |
Block direct airflow |
|
Typical usage |
Between ISO 5 weighing room and ISO 8 warehouse |
Between two auxiliary ISO 8 storage rooms |
A Dynamic Passbox is more than just a "material transfer box" - it is a critical contamination control solution in modern cleanroom design, especially in the pharmaceutical sector, where every mistake can impact end-user safety.
2. Why is a Dynamic Passbox essential in pharmaceutical production?
In pharmaceutical manufacturing, controlling cross-contamination and maintaining cleanliness is a mandatory requirement under GMP, EU-GMP, and WHO-GMP standards. Every material transfer point between functional areas poses a risk of airflow disruption, carrying dust, microbes, or contaminants. That’s why Dynamic Passboxes are nearly mandatory in modern pharma facilities.
1. Cross-contamination control according to GMP
GMP requires clear separation between production areas, especially when materials are transferred from one room to another. The Dynamic Passbox acts as a final-stage filter, ensuring materials are cleaned of dust and microorganisms before entering the next zone, preventing contamination of other batches.
2. Maintain pressure differential - stabilize airflow
Unlike Static Passboxes, Dynamic types feature recirculating blowers and filters, which:
- Limit pressure loss between cleanrooms.
- Prevent reverse airflow when doors are opened in sequence.
- Maintain correct cleanroom zoning (e.g., ISO 8 → ISO 7 → ISO 5).

3. Minimize transfer of microorganisms and dust
Dust, bacteria, and particles can cling to packaging, tools, and equipment. Transferring them between rooms without treatment poses a high risk of product contamination. The Dynamic Passbox performs pre-cleaning - a vital buffer for quality control.
4. Reduce manual handling errors
Manual transfer often leads to:
- Opening both doors simultaneously (violating airlock principles).
- Exposing materials to uncontrolled environments.
- Skipping proper surface cleaning.
With interlocking doors, open-door alarms, and clean airflow, the Dynamic Passbox ensures a more automated and safer process.
See more: Pass Box Qualification in Pharmaceutical Cleanrooms: Ensuring Precision and Compliance
3. Benefits of using Dynamic Passbox in pharmaceuticals
Implementing a Dynamic Passbox in a pharmaceutical production line isn’t just about compliance - it offers tangible benefits for operation, compliance, and economic efficiency.
Operational benefits: Process and cleanliness optimization
- Continuous clean airflow: Integrated HEPA/ULPA filtration cleans the air inside the chamber, preventing contamination.
- Stabilizes cleanroom class: Prevents airflow reversal and maintains pressure differentials across ISO zones.
- Speeds up material transfer: Reduces delays compared to manual handling, ideal for continuous production setups.
Compliance benefits: Easier audits and certifications
- Meets GMP/PIC/S requirements: Demonstrates robust cross-contamination control during inspections.
- Equipped with interlocks and alarms: Enhances safety and prevents common operational errors.
- Optional UV lights, pressure gauges, and filter timers: Adds value and audit-friendliness.
Economic and safety benefits: Product and brand protection
- Minimizes cross-contamination risk: Captures even tiny microbes to safeguard entire production batches.
- Reduces recall risks: Airflow control failures can lead to massive financial losses, especially with injectables or antibiotics.
- Long-term cost efficiency: Compared to the risk and consequences of contamination, investing in a Dynamic Passbox is cost-effective.
4. Where is the Dynamic Passbox suitable within a pharmaceutical facility?
Not every area in a pharmaceutical plant requires a Dynamic Passbox. However, in sensitive transfer zones or areas with different cleanliness grades, this device is practically essential to ensure contamination control and GMP compliance. Below are three of the most common application areas:
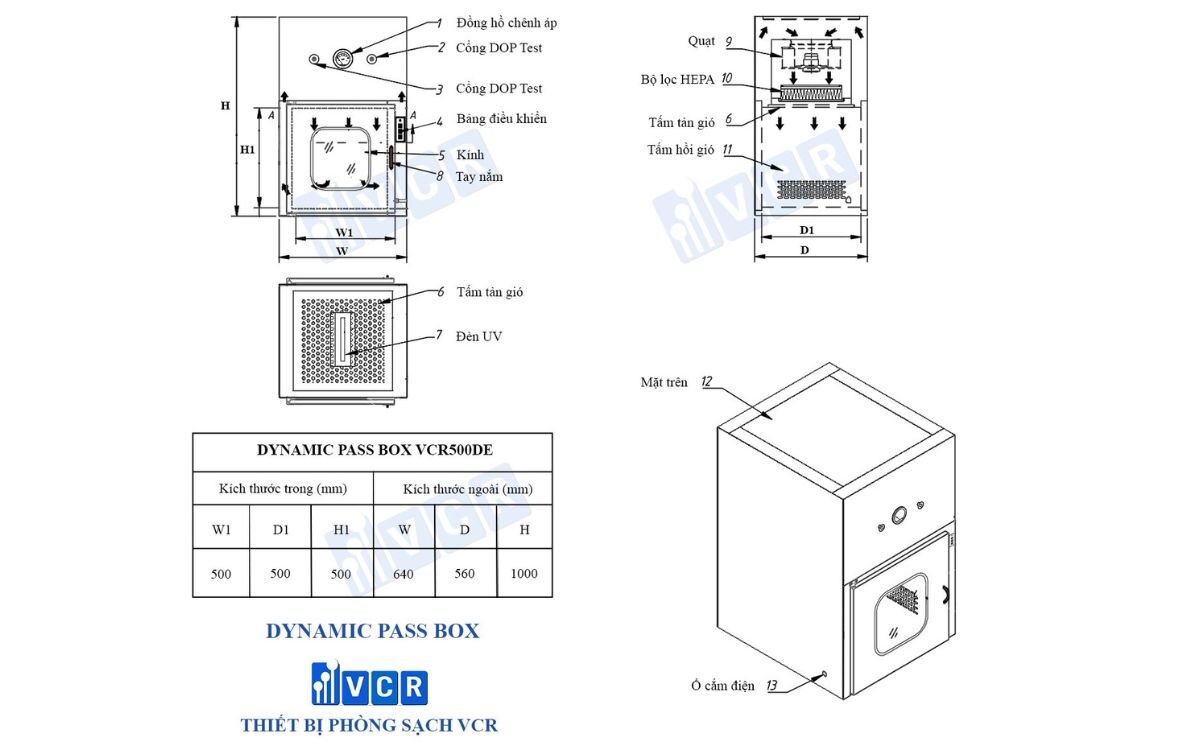
Weighing Room (for raw materials)
This is where active pharmaceutical ingredients (APIs, excipients, etc.) are handled in open form and prone to dust dispersion or reverse contamination.
- The Dynamic Passbox allows materials to be transferred in and out while maintaining proper positive or negative pressure as per design.
- Combined with HEPA filtration and pressure differentials, the unit prevents airflow reversal, reducing the risk of cross-contamination between production stages.
Filling and Packaging Areas
This is the final stage, where the product comes into direct contact with packaging. Even a single dust particle can compromise the entire batch.
- The Dynamic Passbox ensures the air inside the transfer chamber maintains cleanliness levels of ISO 5-7.
- Often paired with 2- or 3-door interlock systems to control movement strictly.
Airlocks Between Clean Zones (e.g., ISO 8 → ISO 5)
When transferring materials between zones with different cleanliness levels, the Dynamic Passbox acts as a safety buffer:
- It pre-cleans the surface of items to prevent dust or microbes from being transferred from a lower to a higher ISO class.
- It maintains pressure differentials and clearly separates cleanroom zones.
See more: Customized Passbox for Specialized Food Manufacturing Processes
5. Key Criteria for Selecting a Dynamic Passbox
Choosing the right Dynamic Passbox ensures operational efficiency, GMP compliance, and optimized investment and maintenance costs. Here are the main criteria to consider:
1. Filter Type and Cleanroom Compatibility
- HEPA (High-Efficiency Particulate Air): ≥ 99.97% efficiency at 0.3 microns - suitable for ISO 7-8.
- ULPA (Ultra Low Penetration Air): ≥ 99.9995% efficiency at 0.12 microns - recommended for ISO 5-6.
- Filters must meet EN1822 or equivalent standards, with test certification provided.
2. Construction Material
- Stainless Steel 304: Corrosion-resistant and suitable for most pharma cleanrooms.
- Stainless Steel 316L: Higher chemical resistance - ideal for areas handling potent ingredients.
- Polished surfaces and rounded edges: Reduce dust accumulation and ease cleaning, critical for audits.

3. Safety and Automation Features
- Door Interlock System: Prevents both doors from being opened simultaneously.
- Visual and audible alarms: Warn operators of incorrect use or airflow failure.
- Pressure sensors and airflow timers: Ensure consistent and accurate operation across shifts.
4. Maintenance and Filter Replacement
- The design should allow external filter replacement without disassembling the unit.
- Options for usage timers or flow reduction alerts are recommended for proactive maintenance.
5. Advanced Options
- UV Sterilization Lamps: Assist in surface decontamination of items.
- BMS or IoT Connectivity: Enables remote monitoring of equipment status.
- Custom sizing: Adaptable to specific airlock zones or facility layouts.
6. Frequently Asked Questions about Dynamic Passboxes in Pharmaceuticals
Is a Dynamic Passbox mandatory in a GMP-compliant pharmaceutical facility?
There is no absolute mandate, but Dynamic Passboxes are highly recommended at material transfer points between areas of differing cleanliness levels (e.g., ISO 8 to ISO 5), or in critical zones like weighing and packaging. Their use helps facilities meet cross-contamination control criteria during GMP audits.
Should I use a Dynamic Passbox or an Air Shower in a weighing room?
These serve different purposes:
- Air Showers clean personnel before they enter the cleanroom.
- Dynamic Passboxes clean items, tools, and packaging during material transfers.
In weighing rooms, where materials are exposed, using both is recommended to minimize contamination risks.
Does a Dynamic Passbox require regular maintenance?
Yes. To ensure performance and cleanroom compliance:
- HEPA/ULPA filters must be inspected and replaced periodically (usually every 6-12 months, depending on usage).
- Fans, sensors, and electronics also need routine cleaning and calibration per internal SOPs.
Proper maintenance extends the lifespan of the unit and prevents costly production interruptions.
7. Need Help Choosing the Right Dynamic Passbox for Your Pharmaceutical Facility?
A Dynamic Passbox is more than a device - it is a critical contamination control solution that supports GMP, PIC/S, and EU-GMP compliance from the design stage. Selecting the right model, location, and configuration ensures long-term safety and efficiency.
VCR - A leading provider of GMP-compliant Dynamic Passboxes in Vietnam
With over 10 years of experience in cleanroom equipment design and installation for the pharmaceutical industry, we offer:
- Expert consultation on the right Passbox for each production area
- Custom sizing and feature integration according to your layout
- Detailed technical drawings, documentation, and GMP audit support
Contact us today for a free quote and expert consultation:
Hotline: 090.123.9008
Email: [email protected]
Website: https://passbox.vn/
Diep VCR