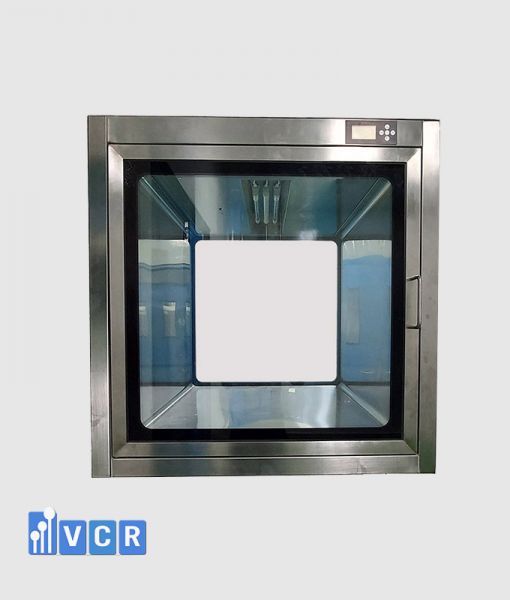
In pharmaceutical production, Pass Box is a mandatory device to prevent cross-contamination and maintain clean air flow. Choosing a GMP-certified Pass Box supplier for pharmaceutical factories helps businesses meet EU-GMP, WHO-GMP, PIC/S standards and ensure product quality.
- 1. Why is the Pass Box a mandatory device in GMP pharmaceutical factories?
- 2. What are the criteria for selecting a GMP-compliant Pass Box?
- 3. Common Pass Box types for GMP pharmaceutical factories (EU/WHO)
- 4. VCR - A Specialized Supplier of Pass Boxes for the Pharmaceutical Industry
- 5. Delivery - Installation - Maintenance according to GMP Standards
- 6. Frequently Asked Questions about Pass Boxes in GMP Pharmaceutical Facilities
- 7. Free Consultation for GMP-Compliant Pass Box Selection
1. Why is the Pass Box a mandatory device in GMP pharmaceutical factories?
In pharmaceutical manufacturing, controlling cross-contamination and separating material transfer routes is a fundamental principle to ensure product quality and comply with GMP regulations. This is exactly why the Pass Box (cleanroom transfer hatch) is considered a mandatory device in all GMP-compliant facilities-regardless of whether it's under EU-GMP, WHO-GMP, or PIC/S standards.
Primary functions of Pass Box in pharmaceutical factories:
- Transfers materials between areas with different cleanliness levels without affecting airflow, pressure balance, or room cleanliness.
- Minimizes direct door opening between rooms-one of the main causes of contamination and pressure instability.
- Integrated interlock and decontamination features (UV, ozone, etc.) to enhance safety when transferring potentially contaminated materials.

Reference from GMP standards:
According to Annex 1 of EU-GMP for sterile production:
"The transfer of materials, raw materials, and packaging should be carried out through intermediary equipment such as Pass Boxes to prevent microbiological or particulate contamination."
Similarly, WHO-GMP recommends:
"Areas with different cleanliness grades must have transfer devices to limit environmental exposure between rooms."
Conclusion: The Pass Box is not merely a support device, but a required component in GMP-compliant cleanroom design-especially in critical areas like:
- Raw material weighing rooms
- Filling and packaging zones
- Material airlocks
2. What are the criteria for selecting a GMP-compliant Pass Box?
A Pass Box suitable for pharmaceutical production must not only operate reliably but also meet the strict requirements of GMP standards, particularly in materials, structure, cleanability, and documentation traceability.
Key selection criteria for Pass Box in pharmaceutical cleanrooms:
|
Criteria |
Requirement Description |
|
Material |
Use stainless steel 304 or 316, non-corrosive, smooth finish, dust-resistant, easy to clean, and resistant to cleaning agents commonly used in GMP environments. |
|
Construction |
Seamless, monolithic design with rounded corners to eliminate bacteria traps and ease cleaning. |
|
Interlock system |
Equipped with mechanical or electronic interlock to prevent both doors from opening simultaneously-avoiding pressure loss and contamination. |
|
Integrated features |
Options to add UV light for disinfection, differential pressure gauges, or mini HEPA fans for high-decontamination zones. |
|
Documentation |
Must include Certificate of Quality (CQ), Certificate of Origin (CO), technical drawings, user manual, and SOPs for cleaning and maintenance. |
Notes for selecting Pass Box for pharmaceutical environments:
- Cleanrooms with ISO 5-7 or Grade A/B should use models with electronic interlock and integrated UV.
- Supporting areas such as material storage or washing zones can use basic mechanical Pass Boxes but must still meet structural and material standards.
A GMP-compliant Pass Box is not only about the device itself, but also its ability to meet validation, traceability, and procedural compliance.
See more: Air Shower Pass Boxes in Cleanrooms
3. Common Pass Box types for GMP pharmaceutical factories (EU/WHO)
In a GMP setting, no single Pass Box fits all applications. Selection depends on the risk level, cleanliness class, and the specific area within the production process.
Below are three common types of Pass Boxes and their typical applications:

Mechanical Pass Box
- Used in supporting areas, material storage, or zones without strict contamination control.
- Equipped with mechanical interlock, easy to install, cost-effective.
- Commonly used where sterile manufacturing is not directly involved.
Semi-automatic Pass Box
- Suitable for central zones such as airlocks, packaging, or finished goods storage.
- Comes with electronic interlock and visual indicators; optional UV light.
- Widely adopted in WHO-GMP compliant facilities.
Electronic/Dynamic Pass Box
- Designed for critical areas such as weighing rooms, filling zones, aseptic processing, or ISO 5-6 areas.
- Features HEPA-filtered air circulation, smart interlock, display panel, and operation history tracking.
- Preferred in facilities following EU-GMP or PIC/S standards.
Comparison table: Pass Box types for GMP compliance
|
Criteria |
Mechanical Pass Box |
Semi-automatic Pass Box |
Electronic / Dynamic Pass Box |
|
Area of application |
Supporting areas, storage |
Central zones, airlocks |
Aseptic rooms, weighing, filling areas |
|
Cleanroom class suitability |
ISO 8-9 |
ISO 7-8 |
ISO 5-6 |
|
Type of interlock |
Mechanical |
Electronic |
Smart electronic |
|
UV / HEPA integration |
No |
Optional |
Yes (upon request) |
|
Installation complexity |
Low |
Medium |
High - requires technical survey |
|
GMP compliance level |
WHO-GMP (basic) |
Full WHO-GMP |
EU-GMP / PIC/S |
|
Cost |
Low |
Medium |
High - but high-performance |
Choosing the right Pass Box not only ensures GMP compliance, but also reduces contamination risks, minimizes maintenance costs, and improves operational efficiency.
4. VCR - A Specialized Supplier of Pass Boxes for the Pharmaceutical Industry
When selecting equipment for pharmaceutical plants-especially for areas requiring strict GMP compliance-you need more than just a supplier. You need a partner who understands the standards, validation processes, and actual usage contexts.

VCR - A trusted solution for pharmaceutical facilities
Over 10 years of experience in cleanroom systems for pharma
VCR has supported many factories in Vietnam in achieving compliance with EU-GMP, WHO-GMP, and PIC/S, particularly in:
- Cleanroom layout design
- Selection of GMP-compliant equipment
- Preparation of technical documentation for validation
Customized Pass Boxes for each area
Every zone-whether it’s the weighing room, filling area, airlock, or packaging room-has its own requirements for:
- Cleanliness classification
- Interlock type
- Materials and optional integrations (UV, HEPA, sensors, etc.)
VCR provides tailor-made design, fabrication, and on-site installation of Pass Boxes that match each plant’s layout and GMP goals.
Complete technical documentation - Ready for validation
All equipment supplied by VCR includes:
- CQ, CO, technical drawings, and SOPs for use and maintenance
- Configuration documentation and factory test reports
- Guidance for QA teams on preparing IQ/OQ validation documents
See more: A Comprehensive Pass Box Cleaning Guide
5. Delivery - Installation - Maintenance according to GMP Standards
A GMP-compliant Pass Box depends not only on design and materials but also on the installation process, operational setup, and long-term maintenance. VCR offers end-to-end services to ensure every device operates safely and correctly from day one.
Nationwide delivery - Project-timed scheduling
- Direct delivery to any project site in Vietnam
- Flexible coordination based on the phases of your project
- Equipment is carefully packed (dust-proof and shock-resistant), with pre-inspection guidelines
On-site installation - Optimized for cleanroom conditions
- Skilled technicians install Pass Boxes according to proper protocols, minimizing contamination risks
- Adjustments made for wall panel height, door thickness, power sources, and existing interlock systems
- Optional support for interlock testing, airflow validation, and pressure differential checks

Scheduled maintenance - Aligned with QA/QC protocols
- Maintenance plans include inspections, cleaning, and component replacements
- Service records provided for GMP audits or supplier evaluations
- Optional recalibration for sensors, UV lights, and HEPA fans
Genuine replacement parts - Always in stock
- Wide inventory of glass, gaskets, hinges, handles, sensors, interlocks, UV ballasts, and more
- Guaranteed OEM parts, model-specific, and easy to replace on-site
- Maintenance diagrams available to support in-house QA teams
With a professional maintenance system, your Pass Box will remain GMP-compliant and reliable throughout its lifecycle.
See more: UV light in pass box
6. Frequently Asked Questions about Pass Boxes in GMP Pharmaceutical Facilities
Is interlock mandatory for Pass Boxes in pharma plants?
Yes. In pharmaceutical manufacturing, interlocks is essential, especially in key areas like weighing or filling rooms. It prevents both doors from opening simultaneously, maintaining pressure balance and preventing cross-contamination-critical for GMP compliance.
Are certifications required for Pass Boxes in GMP facilities?
Yes. Pass Boxes must be delivered with CQ (Certificate of Quality), CO (Certificate of Origin), and complete technical documentation including drawings, user manuals, and SOPs. These documents are essential for QA/QC validation.
Can Pass Boxes be customized in size?
Yes. VCR offers custom fabrication based on wall thickness, door type, table height, and site-specific conditions-ensuring compliance with GMP layouts.
Is periodic maintenance necessary?
Absolutely. Especially for models with UV lights, HEPA fans, or door sensors, regular inspections and calibration are required to maintain functionality and meet GMP standards during audits.
Should I choose mechanical or electronic Pass Boxes?
- Mechanical Pass Boxes are suitable for support zones (e.g., storage, corridors).
- Electronic or semi-automatic models should be used in clean-critical zones such as weighing, aseptic areas, or airlocks. The stricter the cleanliness requirement, the more advanced the Pass Box should be.
7. Free Consultation for GMP-Compliant Pass Box Selection
Are you designing a GMP-certified pharmaceutical plant and need Pass Boxes tailored to each area?
Or are you a QA or engineer preparing validation documents-but unsure which Pass Box model to choose?
VCR is ready to assist you:
- Expert guidance on selecting the right model based on ISO Class 5-8 requirements
- Supply of catalogues, technical drawings, and sample SOPs aligned with GMP protocols
- Support with preparing CQ, CO, and technical documentation
Hotline: 090.123.9008
Email: [email protected]
Website: https://Pass Box.vn
Diep VCR