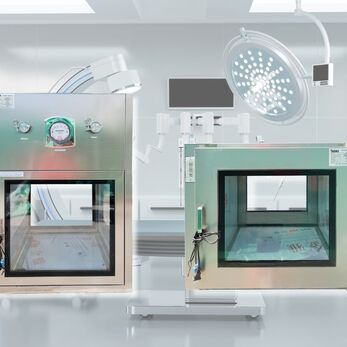
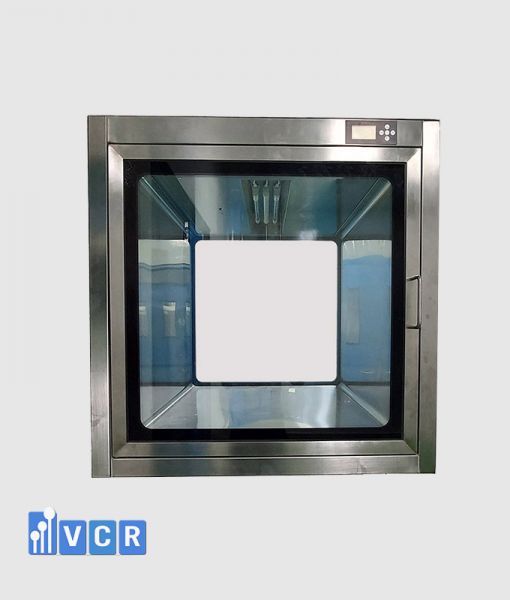
In the intricate realm of pharmaceutical cleanrooms, the strategic use of pass boxes becomes pivotal to prevent cross-contamination, maintain ISO classifications, and uphold product and process integrity.
Pass box, also known as transfer hatches or SAS passes, act as barriers between areas with varying cleanliness levels, facilitating the controlled movement of materials.
Types of Pass Boxes
1. Static or Passive:
For material transfer within areas of the same cleanliness class, featuring interlocked doors and UV light for controlled decontamination.

2. Ventilated:
Designed for transferring materials between classified areas or between classified and non-classified zones. Equipped with air-sweeping HEPA filters to ensure a clean environment.
3. Biological:
Specifically tailored for transferring materials requiring biological neutralization. Incorporates a bio-decontamination system with filtered air and hydrogen peroxide disinfection.
See more:
Pass Box for Pharmaceuticals Cleanroom
Pass Box Qualification Process
Airflow Velocity Test:
Documents average airflow velocity within the pass box. Utilizes instruments like hot wire anemometers or vel-grids, maintaining an average airflow velocity of 0.45 m/s +/- 20%.
Airflow Visualization Test:
Reveals actual airflow patterns within the pass box using non-contaminating items like visible vapor sources or thread streamers. Ensures smooth airflow displacement.

See more: Validating pass box cleanroom
HEPA Filter Integrity Test:
Confirms the absence of leaks in the filter system. Involves introducing aerosol challenges upstream of the filter and scanning downstream to detect any leaks.
See more: Functions of dynamic pass box
Particle Concentration Inspection:
Determines actual particle concentration within the pass box during testing. Particle counters assess airborne particulate cleanliness class compliance for a grade A environment.
Pass box qualification aligns with principles from ISO 14644 and related standards, ensuring consistent equipment performance. While a standard methodology for pass box qualification doesn't exist, adopting principles from established standards provides a robust framework.
Precision in qualification involves meticulous testing, adhering to rigorous guidelines, and embracing a commitment to pharmaceutical cleanliness standards. As the pharmaceutical industry evolves, pass box qualification remains a critical component in ensuring sterile and compliant cleanroom environments.